News
Material extraction and synthesis of electrodic materials
29/04/2025
Lithium-ion batteries proved to be one of the most efficient energy storage solutions, widely used for applications such as electric vehicles [EVs] and renewables. Despite faltering economies and material shortages, more than 40 million EVs are expected on the EU’s roads by 2030. With mass electrification of transport, there will be an important demand for batteries and the materials they are made from. Despite an incentivising regulatory context at EU level, in 2023 Europe was home to only 1% of the production of key battery raw materials. European carmakers and battery cell producers are already fret about volatile prices and limited supplies of cathode and anode materials. But with EV fleet number growing on the EU’s roads, the volumes of End-of-life [EoL] batteries will considerably increase in the near future. And so will the pressure to recycle closer to home, recovering key components and mitigating the supply chain risks.
Conventional recycling methods often require high consumption of energy and reagents. But electrochemistry offers a promising green alternative method, enabling selective recovery of Li without the need for chemical extracting compounds.
Selective electrochemical extraction of lithium
Researchers at Chemistry Department of Sapienza University of Rome [UoS] tested various electrochemical parameters to optimise Li extraction from both commercial cathode material and the black masses [BM] obtained by the mechanical pre-treatment operations conducted in work package [WP] 4.
The electrochemical tests on high-purity commercial cathode materials [LiMn2O4, LiCoO2 and LiNi1/3Mn1/3Co1/3O2] allowed a selective extraction of Li up to 98 %. UoS researchers later applied the same electrochemical process on the black mass recovered by the mechanical pre-treatment, obtaining a lower Li extraction yield of 82 %. This decreased yield was mainly attributed to the presence of conductive carbon which undergoes a simultaneous electrochemical oxidation. Analysis of the resulting electrolyte at the end of delithiation experiments showed negligible extraction of cobalt (Co), nickel (Ni), and manganese (Mn). This demonstrated the method’s ability to selectively extract Li without causing the dissolution of other cathode elements.
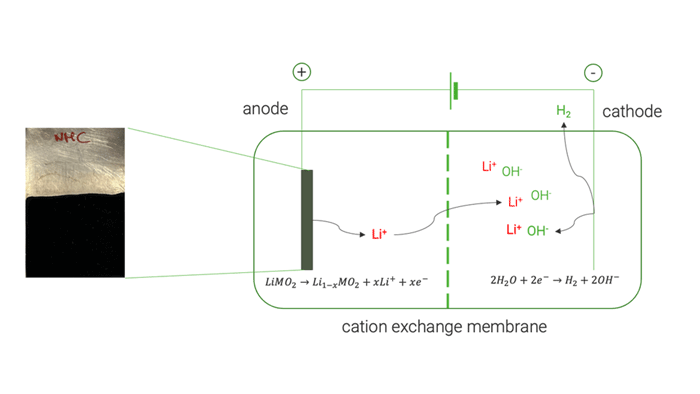
Cation exchange membrane | © Univ. of Sapienza
The optimised electrochemical conditions applied on the BM were replicated in a two-chamber cell to facilitate the separation and concentration of extracted Li on the cathode side, achieving approximately 85 %. The resulting solution was used to crystallise LiOH, resulting in a mixture of LiOH and LiOH·H₂O, with a purity exceeding 99.5 %.
Synthesis of reduced graphene oxide (rGO) and Li-Mn rich cathode material
The direct synthesis of high-value products from EoL LIBs, bypassing the complex and expensive separation of different metals, can be achieved through a competitive recycling strategy. The simultaneous synthesis of reduced graphene oxide (rGO) and lithium-manganese-rich (Li1.2Mn0.55Ni0.15Co0.1O2 – LMR) cathode material from EoL LIBs represents a promising approach to enhance the economic feasibility of hydrometallurgical recycling processes by producing high-value-added advanced materials.
UoS researchers developed an innovative recycling process to directly synthesise a layered LMR cathode and rGO from EoL LIBs. The proposed recycling strategy relies on the application of Hummers’ method to process the electrodic powder delivered by pilot-scale mechanical pre-treatment of mixed Li-ion batteries. The Hummers’ method has allowed for the quantitative extraction of metals from the electrodic powder and the production of GO without any metal impurity. The resulting solution contains the metals from the cathode materials (Co, Ni, Mn) with a significant amount of manganese from the KMnO4 used in the Hummers’ method. From such consideration stems the idea to synthesise LMR cathode material.
UoS researchers synthesised GO using the black masses obtained from three distinct EoL LIB pretreatments performed in WP4. The effects of thermal, mechanical and mechanochemical pretreatments were evaluated using the widely adopted Hummers’ method for graphene production. The study conducted by UoS researchers examined both pristine black masses and those subjected to metal removal via conventional acid leaching, a process frequently employed in LIB recycling to extract metals from cathode materials. This metal removal procedure had a notable effect on introducing oxygen functional group defects across all samples. A synergistic effect was particularly evident in the mechanochemically treated black mass, where acid leaching increased the GO yield significantly. This remarkably high graphite conversion to GO underscores the importance of selecting appropriate pretreatment methods for EoL LIBs, particularly when aiming to integrate advanced materials like GO into the recycling process.
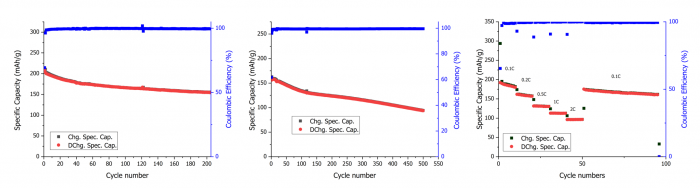
Cycling performance of LMR synthesised from thermally treated black mass: (a) Galvanostatic cycling at 0.1C, (b) Galvanostatic cycling at 1C, and (c) Rate capability performance with the current increasing from 0.1C to 2C every 10 cycles | © UoS
Recovery of Ni/Co materials by solvometallurgical route
RHINOCEROS is a research project that aims to o create economically and environmentally sustainable methods for reusing and recycling LIBs. The project focuses on developing cost-efficient, flexible and eco-friendly processes to recycle all materials in LIBs, including metals, graphite, fluorinated compounds and polymers.
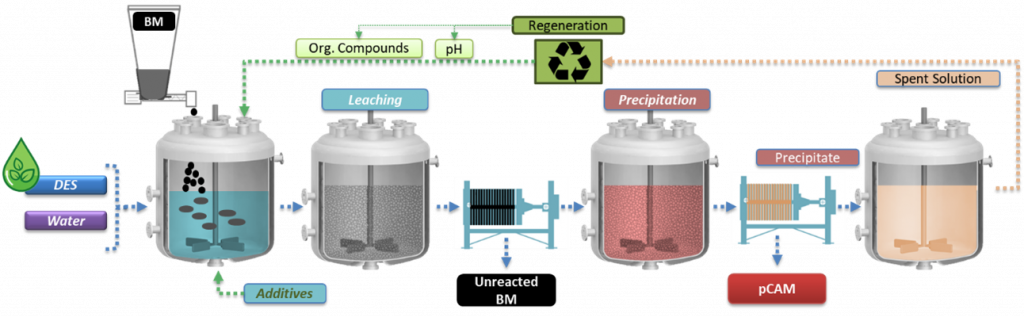
Flowsheet of the leaching, precipitation and regeneration process | © TEC
After studying the effects of the pre-treatment processes applied in WP4 to generate BM, researchers at TECNALIA [TEC] tested various solvometallurgical routes to extract critical metals like Ni, Mn and Co from LIBs under mild conditions. They established optimal process conditions to achieve leaching and precipitation efficiency along with high purity of the produced precursor of cathode active materials (pCAM) under mild conditions. This approach also enhances the recyclability of the extractant.
After characterising all the input materials received from the pretreatment operations, TEC research group analysed also the obtained leachates. The leaching process was optimised at laboratory scale by studying the effects of various parameters such as leaching time and temperature, stirring, waste/liquid ratio and additives.
The solvometallurgical process for recycling end-of-life [EoL] battery BM has successfully developed high-yield, selective systems, validated across various NMC chemistries and black mass pretreatments, achieving over 95 % leaching efficiency. Controlled precipitation delivered up to 95 % precipitation efficiency, producing precursors with more than 99 % purity, meeting project targets. Moreover, researchers have confirmed the scalability of the solvometallurgical process to large-lab testing without performance loss. To address the costs of this process, TEC researchers have also investigated alternatives to reuse and recycle the deep eutectic solvent [DES] leaching spent solution, reporting its dependence on the properties of the BM feed.
The residue after leaching – the graphitic anode material – has been purified via hydrometallurgical routes, underscoring the difficulty of removing the most refractory phases resistant to harsh leaching conditions.
Optimised recovery of materials from low concentration waste streams
A different task in the refining work package, led by LEITAT and TEC, aims at the recovery of low concentration materials from leachates and effluent streams produced in previous refining tasks. It is divided in three subtasks, each exploring different membrane-based technologies to achieve a zero-waste strategy for recovering remaining elements.
The objective of the first subtask is to develop new extractants based on ionic liquids or DESs, to be used within novel Polymer Inclusion Membranes (PIMs), for the recovery of Li from solvometallurgical refining final streams. The team firstly analysed the SoA on Li extraction strategies, selected, synthesised and characterised different potential extractants based on hydrophobic deep eutectic solvents (HDESs). Selected HDESs were synthesised and sent to the next subtask devoted to PIM fabrication and testing using different ILs and/or synthesized HDESs. These membranes represent an innovative technology that effectively adapts to the specificities of each input stream, enabling optimal recovery of valuable metals present in the waste generated by processes carried out. In the this subtask, the PIMs yielded the following recovery rates:
- Co and Mn: efficiencies close to 80 %
- Ni: 60 % recovery
- Li: only 19 %, but exhibiting a high flux rate of 1.2 mol/m²·h, suggesting potential for larger lithium recovery.
Finally, in the last subtask, researchers developed a three-chamber electrochemical process for the extraction, concentration and recovery of lithium in the form of lithium carbonate from solvometallurgical final streams. Different ion-exchange membranes were characterised and lithium carbonate precipitation step was studied and optimised. They tuned the overall process conditions achieving more than 70 % Li extraction from leachate to catholyte and high lithium carbonate precipitation yields (>70 %) with high purity (>94 %).

Three-chamber electrochemical cell | © TEC
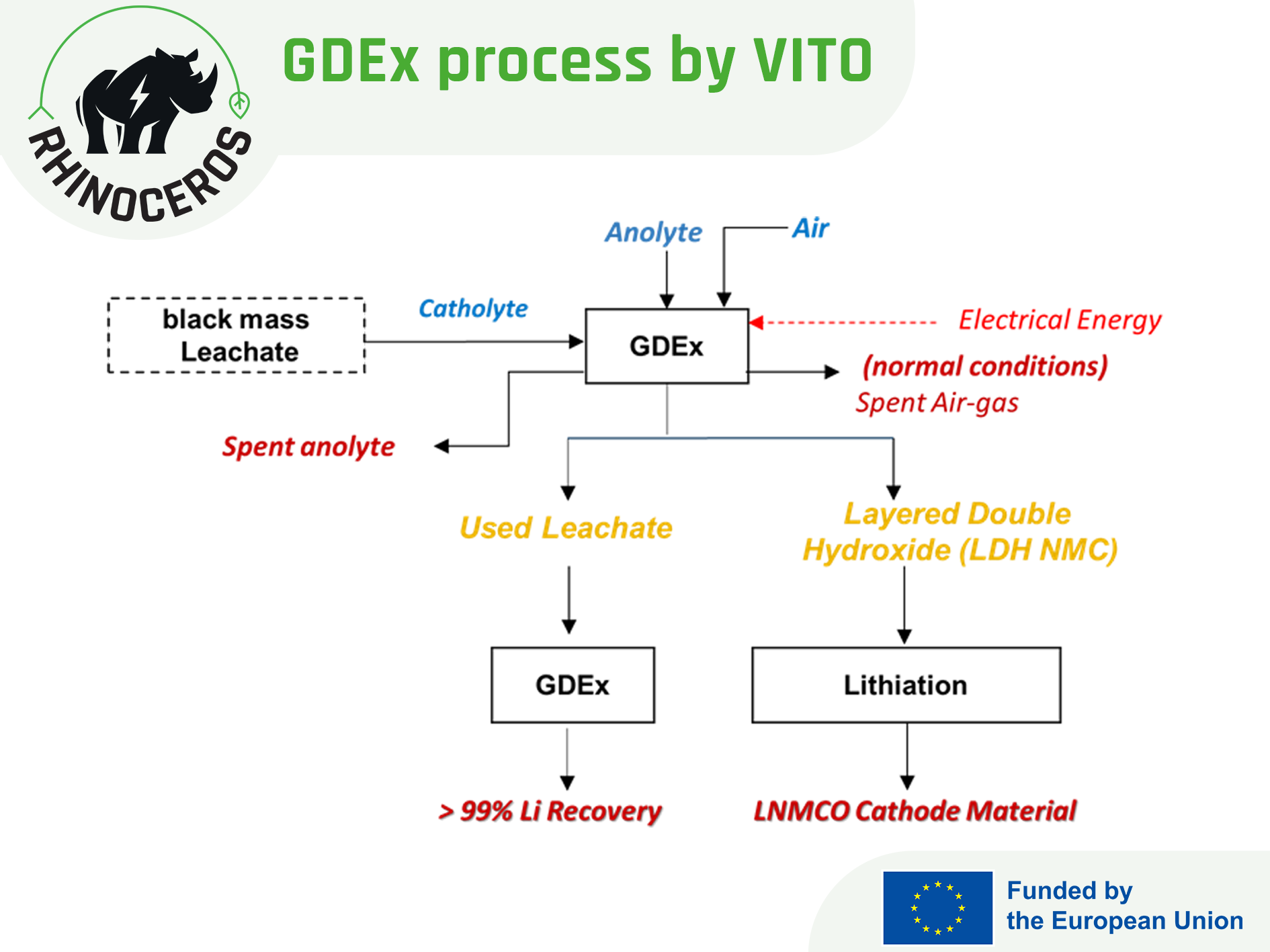
Extraction of Ni/Co/Mn/Li with GDEx
Gas-diffusion electrocrystallisation [GDEx] is an innovative electrochemical process used to recover metals from complex fluids, such as leachates, industrial waste streams and organic solutions. This innovative process has been successfully demonstrated in multiple research projects, proving its capability to recover valuable metals while operating under low-cost and environmentally friendly operating conditions. The GDEx process has achieved Technology Readiness Level [TRL] 3 for the recovery of Co and Mn from synthetic solutions and attained TRL 4 in treating black mass leachates. One of the multiple advantages of this electrochemical process is its capacity to recover Ni, Co and Mn simultaneously, with the flexibility to manipulate operational conditions to obtain targeted products.
Specifically for the battery recycling sector, the GDEx process offers various advantages over conventional battery recycling methods:
- High recovery efficiencies, exceeding 90 % for Co and Mn and over 90 % for Li, with potential for further optimisation.
- Low energy consumption: less than 20 kWh per kg of recovered material, which reduces the operational costs significantly.
- Minimal chemical input: the process relies primarily on air and in-situ synthesised oxidising/reducing agents, ensuring high material efficiency and circular use of non-hazardous chemicals.
- Mild emissions, with the possibility to further treat any effluents using conventional wastewater treatment methods.
- Low temperature operation: from room-to-mild temperatures [18 – 70 °C].
- Direct synthesis of functional battery materials, such as Co/Ni/Mn spinels and birnessites, which can be used to manufacture new lithium-ion battery electrode materials and eventually LIBs. This feature eliminates the need for additional refining steps, streamlining the production of new batteries from recycled material.
Within the RHINOCEROS project framework, researchers at VITO have been finetuning the operational parameters of their proprietary GDEx process, additionally testing and validating the recovered materials through the synthesis of high-performance electrodes for next generations batteries, demonstrating therefore the circularity, cost, environmental and social benefits of the solutions developed.
After testing the direct recovery route of Co, Ni, Mn and Li on synthetic solutions with the main objective to finetune the GDEx operating settings, VITO researchers have been implementing the process to the BM samples provided by the pre-treatment operations [ACC and TES] and to the aqueous extractants processed by the hydrometallurgical route explored by UoS and the solvometallurgical route explored by TEC. Additionally, they also documented the testing operations using a 3D phase diagram material library, mapping the formation of different materials based on Co/Ni/Mn concentration ratios from synthetic solutions.
The GDEx process applied on the leachate solution obtained from the BM provided by ACC achieved high recovery rates: 90 % for Ni, 89 % for Mn and 96 % for Co.
On the other hand, GDEx process implemented on the leachate solutions provided by UoS [originally from the BM of TES] results in the metal extraction efficiencies of 98 % for Ni, 68 % for Mn, and 96 % for Co. Sequential GDEx experiments have potential to improve the recovery efficiency by removing the impurities first and then the recovering the targeted metals. GDEx treatment on TEC’s leachate solution required optimisation due to the presence of high content of Cu and Al impurities.
With better results obtained on the lithiated nickel manganese cobalt oxide (LNMCO) material synthesised from the leached black mass provided by ACC [thermal pre-treatment], VITO researchers validated its electrochemical activity as a cathode material for LIBs with coin-type half cells. The recycled cathode material maintained a stable charge-discharge profile up to 50 cycles, with an initial charge-specific capacity of 37 mAh g⁻¹ and a reversible capacity of 26 mAh g⁻¹. Later, a batch with large amount of BM will be treated by GDEx process to optimise the electrochemical performance of the recycled electrode material.
After removing the targeted transition metals Ni, Co and Mn from the leachate solutions of the BMs, researchers used the same solution to extract lithium, recovering >99 % Li from the leachate of ACC BM.
The low-temperature or near-ambient operation condition of GDEx process eliminates CO₂ emissions, ensuring a clean and cost-effective approach to recovery of NMC precursor. This aligns with RHINOCEROS project goals, promoting a circular and sustainable battery materials economy. These results highlight the sustainability and scalability of the GDEx process, providing a carbon-free alternative for battery material production, aligning with global efforts to decarbonize critical raw material supply chains.
© visual: VITO
News





