News
On 12 December 2024, the third edition of the annual workshop of the Cluster Hub “Production of Raw Materials for Batteries from European Resources” took place in Brussels, being co-organised by EU-funded projects RHINOCEROS, CRM-geothermal and CICERO. This third edition, along with an increasing number membership, confirm the hub’s role as a dynamic ecosystem that continues to generate innovations in the European battery materials sector.
The hub’s annual workshop, held as a satellite event of the Raw Materials Week 2024, provided once again a platform for presenting the most promising results from participating projects. Two technical sessions covered the entire battery value chain, from raw materials mining to recycling, while the opening conveniently portrayed the policy, the regulatory and strategic frameworks that support and drive the EU R&I initiatives in the battery sector.
Policy perspectives and supporting mechanisms for the battery sector
Susana Xara, Project adviser on raw materials at European Health and Digital Executive Agency (HaDEA), established the discussions tone, navigating through the insights of the Critical Raw Materials Act [CRMA] and the Net Zero Industry Act [NZIA] and focusing on their contribution to securing a sustainable supply of critical raw materials for the European battery industry.
Wouter IJzermans, BEPA Executive Director, presented the long-term vision and potential revisions of their roadmap, emphasising the importance of policy frameworks and incentives in promoting battery innovation and deployment across Europe.
The presentation of Vasileios Rizos from the Centre for European Policy Studies (CEPS) identified various barriers and challenges emerging from the EU policy framework on batteries, based on inputs from 20 companies across the entire battery value chain, including partners from the BATRAW project, member of the Cluster Hub since 2022. The representative of CEPS concluded with a set of policy messages referring to early dialogue channels established between policy-makers and various stakeholders. Before the legal requirements entry into force, this information exchange on availability of secondary data sets could enable stakeholders to assess the data quality, select suitable sets of information and identify potential data gaps.
Publicly available resources submitted by CEPS:
- Barriers and policy challenges in developing circularity approaches in the EU battery sector: an assessment – CEPS
- Implementing the EU digital battery passport – CEPS
- Compliance with the EU’s carbon footprint requirements for electric vehicle batteries – CEPS
Orchestrating the launch and on-going work of the Cluster Hub, PNO Innovation Belgium [part of PNO Group – leader in innovation and funding consultancy], represented by Dr. Nader Akil, concluded the first session with an overview of all EU funding programmes supporting research, innovation and investment in raw materials production for batteries. Additional to the upcoming funding opportunities and guidance on selecting the appropriate funding opportunities based on the status of technology, Dr. Nader Akil introduced another initiative launched by PNO Group – DIAMONDS4IF. This project supports the preparation of Innovation Fund applications, enabling the transfer of H2020 research results into successful ventures and securing investment funding.
Download Funding Schemes presentation
The third session of presentations commenced with an outline of the main findings of the LIFE DRONE project, which concluded in June 2024. Presented by Lorenzo Toro, process engineer at Eco Recycling, the project demonstrated the feasibility of producing high-quality NMC oxide and graphite from recycled batteries. The innovative process confirmed significant environmental benefits, with a reduction of 59 % in terms of kg CO2 eq. Additionally, one plant is estimated to treat 500 tonnes of batteries/year. The technical-economic evaluation of this industrial plant, with a potential capacity of 500 tons/year, showed a return on investment (ROI) of 31.64 % and a payback time (PBT) of 3.16 years. The analysis indicated that attractive payback times could be obtained even with varying prices for NMC and graphite.
Download LIFE DRONE presentation
RHINOCEROS presenting results of the Electrochemical Li recovery strategy from LIBs black mass
The electrochemical Li recovery from Li-ion battery black mass, investigated in the RHINOCEROS project and presented by Prof. Pier Giorgio Schiavi from Univ. of Sapienza reported Li extraction yields from end-of-life (EoL) LIBs in the range of 82 %, and faradaic efficiency compared to commercial cathode materials (close to 100 %). Researchers have investigated potential causes that could explain the relatively low selectivity ranges of Li and other metals available in black mass. The simultaneous oxidation of impurities found in the black mass can be a justified explanation for the preliminary results obtained. Experimental results show that the extraction percentages for Co, Ni, and Mn remain in very low ranges. When contemplating upscaling scenarios, Prof. Schiavi mentioned the researchers are evaluating an alternative approach that enables the treatment of larger quantities of powder without replicating the manufacturing process of LIB electrodes.
Download RHINOCEROS presentation
The session featured also presentations of other initiatives addressing the batteries recycling topic: Benjamin P. Wilson, Senior Scientist, Hydrometallurgy and Corrosion at Aalto University on behalf of the RESPECT project, Miguel Aguilar, researcher at LEITAT Technological Centre for the BATRAW projects, and Joana Gouveia [Researcher at the Institute of Mechanical Engineering and Industrial Management (INEGI) and America Quinteros [Researcher at LUT Univ.] for the ReLiEF initiative.
When it comes to recycling lithium-ion batteries (LiBs), safety and efficiency are paramount. Classified as hazardous waste under EU legislation, spent LiBs pose significant risks, primarily due to their state-of-the-art (SoA) non-aqueous electrolytes. This complex mixture, which includes conductive salts dissolved in organic solvents and additives, is flammable, volatile, and toxic. By their very nature, the uncontrolled release of these components can harm the environment and endanger workers in recycling plants. Additionally, electrolyte residues in LiB waste streams represent a financial burden for the recycling industry since they are still classified as hazardous waste. Therefore, safely recovering the electrolyte is crucial for developing a secure recycling process.
One promising alternative to traditional methods like vacuum vaporization is supercritical carbon dioxide (ScCO2) extraction. The easily adjustable properties and excellent mass-transfer characteristics of ScCO2 make it potentially ideal for selectively extracting electrolyte components from LiB waste, resulting in purified extraction products. Previous research has demonstrated that non-polar electrolyte solvents like dimethyl carbonate (DMC) and ethyl methyl carbonate (EMC) can be extracted using low-density CO2.
Recent results reported by the research team at University of Chalmers (CHA)have shown that by gradually increasing pressure and temperature conditions, more polar electrolyte components such as ethylene carbonate (EC), and propylene carbonate (PC) can also be successfully extracted. However, selective extraction of solvents remains a challenge, requiring further thermodynamic and kinetic data to optimise the process.
Modelling the extraction behaviour allows the designing of an optimised extraction process that achieves high purity solvents. This high purity enables the recycling industry to either resell the solvents for other uses or even reuse them in battery production, making the process more sustainable and economically viable.
Discover the scientific publication
Work package (WP) 6 partners, notably ECO RECYCLING (IT) team, have completed the first version of the simulations of various hydrometallurgical processes. These tools are essential for the next scaling step since they provide a first version of the material and energy balance. In addition, these simulations make it possible to estimate the time needed to complete a production cycle, to define potential bottlenecks at each step and, above all, to define the necessary equipment and their sizing in order to produce 10kg/day of battery active material.
In parallel with the process simulations, WP6 research team compiled an equipment inventory to assess the potential use of the facilities currently available at JGI-HYDROMETAL (BE), as part of the RHINOCEROS project. This timely inventory will help the partners to identify the additional equipment requirements necessary to properly equip the pilot plant for the scale-up process. Work is underway to best prepare the construction of the future pilot.
Benefits of “offline programming”
Simulation environments have been widely used in robotics for demonstration and planning purposes. This typically takes place within a simulation software or any other platform that can replicate the robot’s dynamics, workspace and surrounding environment, and enable robotic programming. This replication system has proved to be cost- and time-efficient due to a series of advantages: no risk of disrupting the production by removing the robot from the production line, high flexibility allowing infinite number of configurations on a virtual model of the robot, reduced risk of equipment damage due to high predictability of malfunctions. For instance, operational industrial robots can be tested in a simulation environment before deployment. This process is often referred to as “offline programming”.
Researchers at Department of Engineering Sciences, University of Agder have been designing a simulator within a virtual environment to visualise and test various demanufacturing approaches for battery packs, allowing them to collect necessary data such as process duration, disassembly tools – all without the need of physical experiments. This innovative exploration not only streamlines data gathering but can also help identify and remove unforeseen bottlenecks in the disassembly process.
Environment configuration and use case application for battery pack demanufacturing
Using a simulation environment, known for its high-fidelity graphical capabilities, researchers at UiA were able to create a controlled virtual space ideal for visualising complex robotic processes and interactions related to demanufacturing electric vehicle (EV) batteries. The robotic cell design is decomposed across all the subtasks/segments of the disassembly process, with specific consideration to safety aspects and optimised efficiency and accessibility of robotic manipulators.
In order to study in depth and to demonstrate the efficacy of a proposed fully automated demanufacturing line, researchers at UiA meticulously recreated a virtual environment where they simulated the disassembly of a an EV battery pack. This simulation encompasses the entire process from automated discharging to the disassembly of packs into modules, subsequent characterisation, sorting, and finally, the disassembly of modules into individual cells. All elements of the simulation are animated using the simulation platform and a robotic operating system code, providing a holistic view of the potential automation within the demanufacturing process.
For this particular use case, researchers at UiA have calculated the time individually for each disassembly operation, reaching roughly between 12 and 14 minutes for the entire process.
The findings of this research that replicated the complete demanufacturing of EV LiB pack in a virtual, yet realistic industrial setting, illustrate the leverage of automated processes over conventional approaches conventionally relying on manual techniques. The simulation provides estimates for operation time for a given disassembly procedure (disassembly sequence and disassembly process). Upcoming steps will involve AI to generate and optimise the procedures. Additionally, the simulation can identify solutions to minimise human exposure to potential hazards associated with battery disassembly processes. Future in depth and multidisciplinary research is required to optimise the disassembly sequences and process in the simulated environment by training reinforcement learning agents and including a collision avoidance system, to name a few.
Ultimately, the aim of this research is to anticipate the increasing number of EV batteries that will be decommissioned soon, and to ensure a proper management of waste, while recovering all the resources available in clean mobility technologies.
Discover UiA’s previous activities
© Photo: Adobe
Authors: CHA and PNO
An important component of a Li-ion battery (LiB), the electrolyte has a crucial role in the cell performance. The nonaqueous electrolyte is a multicomponent system consisting of a conductive salt, mainly LiPF6 (Lithium hexafluorophosphate – inorganic component), organic carbonate solvents and additives.
Numerous research initiatives are dedicated to the design of the electrolyte composition, aiming to optimized performance, increased safety, lifetime and streamlined costs. However, once a LiB reaches its end-of-life (EoL) stages, the electrolyte receives less attention in the favour of the valuable metals that can be recycled from the cathode: Li, Co, Mn, Ni, Al, Cu. Moreover, due to its volatility, toxicity and high flammability, the electrolyte recycling is less studied. When ignored, the spent electrolyte reacts with water to form fluoride, leading to its uncontrolled decomposition and/or evaporation, and thus generating an imminent environmental risk. Due to its composition that includes organic solvents, the presence of residual electrolyte in the black mass is considered hazardous, and it is often a reason hampering the recycling process.
Researchers at Chalmers University (CHA) ) developed a sub- and supercritical carbon dioxide (sc-CO2) extraction process to selectively recover the electrolyte from spent LiBs. Sc-CO2 extraction technologies are already widely employed in the food, beverage, pharmaceutical, and cosmetic industry. In a nutshell, sc-CO2 forms when CO2 surpasses its critical point at 31°C and 73.8 bar pressure. In this so-called supercritical state, CO2 demonstrates optimal mass-transfer characteristics and can be fine-tuned to alter its physicochemical characteristics by adjusting pressure and/or temperature. However, CO2 proves ineffective as a solvent for high molecular weight polymers and highly polar ionic compounds. However, the addition of a co-solvent or a modifier can significantly improve the solubility properties of sc-CO2, making this alternative a suitable process to selectively extract LiPF6 after solvent removal.
Researchers at CHA investigated different process parameters (pressure, temperature, extraction times) to understand their impact on the extraction behavior of different electrolyte solvents such as ethylene carbonate. Their analysis included both qualitative and quantitative results indicating the composition and the purity. Simultaneously, the research also monitored the process exhaust stream for potential formation of LiPF6 decomposition products. The findings showed that that the non-polar electrolyte solvents like dimethyl carbonate, diethyl carbonate, and ethyl methyl carbonate were successfully extracted using low-density CO2. The more polar electrolyte components such as ethylene carbonate and propylene carbonate were stepwise extracted by gradually increasing the system’s pressure. The developed technology is a game changer not only for electrolyte recycling but also for increased workplace and transportation safety due to removal of the flammable and hazardous substances from battery black mass. The simplicity of the processing design is an additional advantage of the technology.
© Photo credit: Adobe
During the past years, the increase in the use of lithium-ion batteries (LIBs) has become more prominent. An unsurprising trend, whatsoever, due to the widespread and rapid adoption of clean mobility applications, electronic devices, and energy storage systems. Despite their undeniable environmental and social benefits, several challenges lie ahead. In 2022, global lithium demand exceeded the supply despite an 180% increase, IEA reports. Recent communications already forecast the demand of lithium (Li) is expected to soar over the next decade, with mobility accounting for the main consuming market.
The current supply of primary resources is deemed insufficient for the growing demand. Approximately 60% of today’s lithium is mined for battery-related applications, a figure that could reach 95 percent by 2030, McKinsey reports estimate. But this rapid increase in the use of LIBs in EVs will introduce a large quantity of spent batteries in the near future. The alternatives to manage spent batteries include remanufacturing, repurposing and recycling, with the latter one playing a significant role from both ecologic and economic points of view.
Current recycling processes lack selectivity in recovery control and require significant consumption of reagents and energy. The research conducted by the RHINOCEROS partners at Sapienza University of Rome (UoS), Department of Chemistry, aims to develop an electrochemical process for selective extraction of Li from electrodic powder of end-of-life (EoL) LIBs. This concept simulates the charging process of a LIB with an aqueous electrolyte and a cathode material (counter electrode) that facilitates water reduction. The hydroxyls freed by water reduction and the Li + cations deintercalated by the anode will form a LiOH solution.
Using two samples – a commercial powder, respectively one coming from EoL LIBs, and testing the delithiation process using various parametres, UoS researchers obtained:
- Li extraction of 99% from commercial powder
- Li extraction of 82% from waste powder
The lower Li extraction on EoL electrode powders compared to commercial ones is due to the presence of SuperP [a high purity and structured carbon black powder with a moderate surface area for the lithium-ion industry], which oxidises under delithiation conditions.
Discover the scientific publication
Europe stands at a turning point in its journey towards establishing a competitive European value chain for batteries. Important steps have been taken in encouraging battery manufacturing plants, only to mention here the inauguration of the first gigafactory by Northvolt in Sweden. Yet, the market demand for batteries continues to surge, fueled not only by the electric vehicle sector but also by other mobility applications and stationary storage needs. The recently launched Quarterly EU Electricity Market Report Q3 ’23 indicates over 600,000 new battery electric vehicles (BEVs) were registered in Q3 ’23, 36% higher than the corresponding quarter in 2022 and counting for 24% market share.
In response to these record demands, the European batteries research and innovation (R&I) community has been dedicated to supporting the establishment of this industrial value chain in Europe, aided by public funding, including by the European Union. Various R&I projects under the umbrella of the BATT4EU Partnership (established under Horizon Europe Programme in 2021), RHINOCEROS included, are sharing forces within the Cluster Hub “Production of materials for batteries from European resources” to address common challenges.
Motivated by the global geopolitical developments, the strategic role batteries play in achieving Green Deal objectives and the ever-evolving nature of battery technologies, Europe recognises the critical need for strategic alignment among stakeholders. Replacing the BATT4EU SRIA of 2021 and the Batteries Europe SRA of 2020, the 2024 SRIA outlines key strategic actions that the European Batteries R&I Community will undertake to advance collaborative research projects facilitated by the BATT4EU Partnership. Different from the previous strategic agendas, the 2024 roadmap goes beyond specific chemistries, leveraging also the power of disruptive (digital) technologies to advance research across all battery types, including material science, manufacturing and recycling processes.
The new agenda draws on the roadmaps published by Batteries Europe and Battery 2030+, compiling inputs from numerous European battery experts, offering recommendations on short, medium, and long-term objectives. It emphasises the need for coordinated action not only at the European level but also within national and regional programmes.
The 2024 SRIA points to the following six imperatives which are necessary to set the foundations and support a competitive battery value chain in Europe:
• Ensure that (BATT4EU) research results reach gigafactories and the markets, through pilots, demonstrators and improved decision making aided by digital tools.
• Increase the strategic autonomy of Europe by reducing the reliance on foreign critical raw materials by supporting local and circular supply chains and support research into different battery chemistries, including sodium-ion technologies.
• Improve battery affordability to accelerate the green transition and keep the European industry competitive by improving batteries based on materials that are more abundant and pushing for better integration into end-use applications.
• Improve the flexibility of battery manufacturing and recycling systems to reduce lock-in effects and respond quickly to changes in a rapidly developing industry.
• Implement a safe and sustainable by design framework for batteries, which plays to European strengths, and which will help reduce emissions and use of substances of concern, improve safety and allow for the integration of smart functionalities.
• Support the continuity of excellent European battery research and academic-industrial cooperation by improving access to research facilities and pilot lines, use research projects to build up a skilled workface, and by avoiding gaps in research through continued funding, which will bind talented researchers to Europe.
Interested in finding out more information about the recently released SRIA?
BATT4EU Partnership is organising a webinar on 20 March 2024, between 10:00 and 11:30 Brussels time. The aim is to present the official document and to host engaging discussions with the experts behind this publication who will explain how this document will redefine the dynamics for the European battery sector.
Register here
RHINOCEROS attending Shifting Economy Week
From 21 to 25 November 2023, the city of Brussels hosted the Shifting Economy Week, an annual event dedicated to showcasing transformative projects that aim to pave the way to an economy that is low-carbon, regenerative, and equally circular. The 2023 exhibitors’ line-up included, among other regional stakeholders, our partner Watt4Ever (W4E), industrial partner specialised in the development of innovative solutions for energy storage and management. W4E leveraged its presence at Shifting Economy Week to to raise awareness about the importance of circular economy principles in the context of the battery industry.
During the same event, W4E’s CEO, Aimilios Orfanos, was invited to speak at the BeCircular conference, an event dedicated to presenting concrete examples of circular economy approaches put in place by Brussels-based companies. He shared insights from W4E’s experience in developing second-life battery systems for electric vehicles, emphasising their potential benefits in terms of environmental impact and cost savings. Simultaneously, the CEO also highlighted the challenges faced by the industry in implementing circular business models, including regulatory barriers and market incentives.

RHINOCEROS at its second participation at Circular Wallonia Days
A few days after attending Shifting Economy Week, W4E represented the RHINOCEROS project at the Circular Wallonia Days, held on 13 and 14 December 2023. Centred around advancing the circularity of the batteries value chain, the event brought together stakeholders from academia, industry, and government to discuss strategies for improving the sustainability of battery production, use, and disposal. The focus topics covered recycling technologies, supply chain transparency, and policy measures to support the transition to a circular battery economy.
© Photo credits: Watt4Ever
Despite a different objective, the RHINOCEROS project partners have shown growing interest in the Digital Battery Passport, an initiative of FREE4LIB, a sister project from the Cluster Hub “Production of raw materials for batteries from European resources”. This collaboration shows our commitment to contributing to the European battery community through the exchange of knowledge and experience.
The FREE4LIB workshop had a three-fold objective, including a brief presentation of the preliminary results of the battery passport concept development, the outline of the implementation challenges and potential follow-ups of industrial scale-up, and the clear differentiation between battery second use (B2U) versus recycling. The event drew approximately 50 participants from various segments of the battery value chain, which ensured a comprehensive and multifaceted perspective of the subject matter.
The introductive session presented the FREE4LIB project, briefly highlighting past achievements and focusing mainly on the remaining activities outlined in the workplan. The following session was led by Julius Ott (industrial engineer with expertise in circular economy at Karl-Franzens-Universität Graz). During the past year, researchers at Univ. of Graz worked on finalising data collection and processing related to the development of a data model of the digital passport platform which aims to close the information gap between beginning-of-life (BoL) and end-of-life (EoL) battery lifetime. This interactive session turned out to be an appropriate opportunity for researchers at Univ. of Graz to present the outcomes of their data collection and handling, and to evaluate their relevance within the reality portrayed by the workshop attendees.
Participants, predominantly familiarised with the EU-funded battery projects, confirmed the findings reported by Univ. of Graz. However, they also raised concerns about data sharing. The outcomes of the interactive session, complementing prior research, will serve as valuable guidance for the FREE4LIB project in implementing the battery passport within their project.
For additional background information on the digital passport developed by FREE4LIB, please refer to previous articles.
On Thursday, 16 November, during the 2023 edition of the Raw Materials Week, the twelve EU funded projects that constitute the Cluster Hub ‘Materials for batteries’ gathered for their annual event in Brussels.
The Cluster Hub has been initiated last year during the 7th edition of the Raw Materials Week. The main objective of the meeting was to meet and discuss the latest developments in the participating projects as well as the new challenges and opportunities discovered through the projects’ lifetime. Nader Akil, Operations Manager at PNO Innovation, inaugurated this second edition outlining the motivation behind the hub’s establishment. He underlined the positive reception and sustained interest from various stakeholders keen on joining this initiative.
Discover and/or rediscover the first edition of the Cluster Hub workshop
Co-organised by RELiEF, EXCEED, ENICON and RAWMINA, the event was also the opportunity to welcome the four new members of the Cluster (EXCEED, RAWMINA, METALLICO and CRM-geothermal). the workshop gathered nearly 100 organisations driving the production and the recycling of raw materials for battery applications from primary and secondary resources.
Building on the initial objective of creating an environment that could foster knowledge exchange on different approaches for the recycling and recovery for battery applications, the event focused on three major topics that depict the transversality characterising the projects: the raw materials through research and science, the roles and challenges of industry and market for raw materials, and the raw materials under the scope of sustainability, durability and social acceptance. During this annual meeting, an interactive session led by Anish Patil from TechConcepts and representing the RELiEF project had the objective of Mapping the European battery material recycling landscape – more details to be found below, in the section referring to the interactive session.
Research and science unlocking new opportunities in raw materials
The first session was moderated by Sonia Matencio from LEITAT, representing the RAWMINA project. This session had the objective of discussing the raw materials through research and science, under the scopes of mining, refining, processing as well as the battery data. Sonia introduced this topic under the scope of RAWMINA, explaining the integrated innovative pilot system for Critical Raw Materials recovery from mine waste in a circular economy context. To this end, Christophe Aucher, from LEITAT as well, highlighted the need on an open battery passport system to better reflect and account for any adaptations that might be required due to the changing regulatory landscape.
Sonia welcomed afterward Brecht Dewulf from KU LEUVEN and representing ENICON, who discussed the sustainable processing of Europe’s low grade sulphidic and lateritic Ni/Co ores and tailings into battery grade metals. The idea behind this was to show all the potential of Ni/Co resources for Europe.
Xochitl Dominguez from VITO concentrated her speech on gas-diffusion electrocrytallisation (GDEx), a crucial topic for the projects LiCORNE and RHINOCEROS she works with. GDEx is an electrochemical process of reactive precipitation of metals in solution with oxidising or reducing agents produced in-situ by the electrochemical reduction of a gas, in a gas-diffusion electrode. This was followed by Katrin Kieling from GFZ Potsdam, working there for the CRM-geothermal project and shortly explained the challenges of extracting critical raw materials from geothermal fluids. To conclude this first session, Sandra Pavón from Fraunhofer IKTS explained the demonstration of battery metals recovery from primary and secondary resources through a sustainable processing methodology in the METALLICO project.
Discover presentations from Session 1
Insights from stakeholder perspectives: Interactive session on key EU Policies and priorities
The annual meeting followed its course with an interactive session led by Anish Patil, which scrutinised stakeholders’ perspectives on the Green Deal Industrial Plan, Net Zero Industrial Act, Critical Raw Materials Act and the European Battery Regulation 2023. Mentimeter facilitated this interactive session, engaging the audience to explore how these policies intersect, complement each other, and identify critical measures and incentives for achieving their objectives.
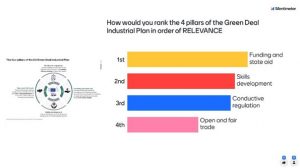
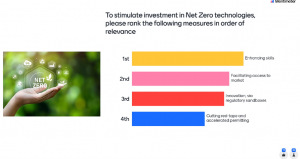
Over 30 persons participated in the live-poll proposed, which results display the priority to be set on funding and state aid regarding ranking the four pillars of the Green Deal Industrial Plan in order of relevance (followed by skills development, conductive regulation, and open and fair trade). Another major topic regarding the stimulation of investment in net Zero technologies, the majority of answers placed the ‘enhanced skills’ as first priority, shortly followed by facilitating the access to the market.
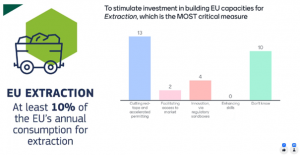
Lastly, the participants were divided regarding the critical measures to implement in the EU to stimulate investment in building domestic capacities for extraction of critical raw materials (CRMs). Although the majority opted for ‘cutting red-tape and accelerated permitting’, approximately half of the answers evoked uncertainty, which emphasised one more time the need to engage with policy makers as external stakeholders in all projects.
Navigating the nexus: industry challenges, market dynamics, social acceptance and sustainability
This interactive workshop was followed by two sessions, which aimed at discussing the challenges and opportunities of raw materials within the frame of industry and market, as well as the social acceptance, sustainability, and durability.
Alan Gonzalez from PNO Innovation Begium, representing LiCORNE, moderated the industry part, whereas Sam Hoefman from RELiEF moderated the last session on social acceptance, sustainability, and durability. Distinguished panellists took the stage to engage in debates on various topics.
Edvarts Emerson, Production and Testing Engineer at Watt4Ever, presented his work on the benchmark depository of 2nd life use of lithium in batteries, acceptance criteria and guidelines, work developed within the RHINOCEROS project. Benjamin Wilson, representing the RESPECT Project, displayed Aalto University’s work advancing efficient, sustainable, innovative and safe battery recycling processes in the EU. Laura Kainiemi from LUT University, representing the RELiEF Project, Konstantinos Komnitsas from the Technical University of Crete (TUC), on behalf of EXCEED, and Vitor Correia from INTRAW for the CRM-geothermal project, collectively debated the role and impact of social acceptance among affected communities, the importance of triggering new dialogues on responsible mining activities, and the joint involvement of regional, national and European authorities, academia, industry partners, and citizens in shaping these initiatives.
A big thank you to all participants for this co-creative and very constructive and inspiring meeting.