News
The challenge of recycling lithium-ion batteries
Current battery recycling is confined to recovering raw materials from the scrap produced by gigafactories. But with the adoption of lithium-ion batteries (LIBs) increasing, a much richer vein will soon emerge, as the first wave of electric vehicles (EVs) reach the end of their lives. And with it, an additional challenge emerges managing the growing waste generated by these energy storage applications. Traditional recycling methods, such as pyrometallurgy and hydrometallurgy, have faced certain hurdles in efficiently recovering valuable materials, mainly due to the presence of the organic components present in the battery binders and separators. Some methods are labour-intensive, whereas others need lots of energy or are environmentally harmful. But each hurdle is another opportunity for innovation.
Optimisation of shredding pre-treatment: TES develops and validates their mechanical process for battery recycling
Leveraging over 20 years’ experience in battery recycling, TES partners oversee the development of a shredding process for batteries using its patented in-house technology. Over the first two years of the RHINOCEROS project, they have been conducting various activities using commercial battery packs, from evaluation and deactivation to pack-to-cell dismantling.
TES have been prioritising safety measures and efficiency, ensuring the system extracts the residual energy stored in batteries, preventing it from going to waste. Following the discharge process, TES applies its proprietary mechano-physical pre-treatment, which involves pre-shredding under an inert atmosphere. This is followed by physical separation techniques to recover polymers, base metals (Al, Cu, Fe), and black mass containing valuable active materials such as Co, Ni, Mn, Li and graphite.
Despite the effectiveness of the current mechano-physical treatment, TES has identified areas for improvement. Moving beyond the state-of-the-art [SoA], TES has optimised its process with a pre-drying step to minimise solvent residues and enhance delamination and separation efficiency. These advancements have led to significant improvements in material recovery and product quality, making the recovered black mass more suitable for downstream hydrometallurgical processing.
- Electrolyte recovery rate: exceeding 95 %
- Polymer recovery rate: exceeding 80 %
- Lithium recovery rate: surpassing 95 %, with the majority retained in the black mass
- Impurity levels of Cu and Al: reduced below 1 %
Optimisation of thermal pre-treatment process
However, reintegration of cathodic and anodic materials into the production chain of new batteries remains a challenge. Thermal pre-treatment of the batteries remains paramount in deactivating, releasing and separating the battery materials. But before reaching the thermal pre-treatment, batteries undergo a sorting process according to the cell type. Discharging is not mandatory prior to the pre-treatment.
ACCUREC [ACC] partners have been modifying their thermal pre-treatment process, including a preceding stage for cell solvent recovery, which places their process beyond the SoA. To explore this technological path, they experimented various parameters altering the working temperature and the pressure of solvent distillation, to address safety concerns and stabilise the background electrolyte. A selective recovery of solvent was possible under specific temperature and pressure conditions achieving high purities >99.6 %. However, additional refining steps are necessary prior to further use in battery manufacturing. ACC partners managed to deactivate batteries completely and produce high-purity black mass [BM] with recovery yields of >95 %, using high temperature pyrolysis and a two-stages screening process.
Their process demonstrates that the distillation process prior to the thermal treatment allows the extraction of solvents used in the cell and streamlines high recovery rates of active material. However, their research concludes that the processing of end-of-life [EoL] batteries remains a trade-off between recycling efficiency and purity of the obtained fractions due to the large number of compounds and lack of control over the starting material.
Electrolyte recycling
The safe and environmentally responsible recovery of electrolytes is crucial in the LIBs recycling process. The SoA non-aqueous liquid electrolytes consist of a conductive salt (lithium hexafluorophosphate, LiPF6) dissolved in a mixture of organic solvents and additives. Many of these components are highly volatile, flammable, and classified as toxic. The current state-of-the-art LiB recycling process begins with the production of black mass, obtained through a series of pre-treatment steps. But uncontrolled evaporation of volatile electrolyte solvents during these stages presents major safety hazards. The research group at Technical University of Chalmers [CHA] have been exploring various processing pressure and temperature parameter settings for their supercritical carbon dioxide [scCO2], with a particular focus on the extraction of ethylene carbonate [EC].
CHA researchers successfully developed and validated the scCO2 process for electrolyte recycling from LIB black mass. The results demonstrated a >95 % overall recovery, with individual yields of >99 % for DMC, EMC, and DEC and 95 % for EC, using specific process conditions. The recovered EC retains its original structural integrity, confirming its chemical stability and purity.
But beyond the high purity and selectivity of carbonates, the developed scCO2 does not require any chemical reagents. Moreover, the used CO2 can be indefinitely recycled within the system, turning it into a clean separation process that eliminates toxic gas during processing.
Reactive milling for black mass production
The research group at KIT received black mass samples from partners ACC and TES. Only the samples from TES were fit to study the mechanochemical transformation of BM, as the ACC supply was already in a reduced state. The BM supplied by TES consists mostly of NMC (lithium nickel manganese cobalt oxides) cathode material and graphite, which was found to slow down the reaction kinetics.
KIT researchers applied their reactive milling on the BM provided by TES to reduce the cathode material to its metallic form and produce water-soluble lithium salts. They optimised the process using three types of mills [two planetary mills of different sizes and a SPEX shaker mill], engaging three types of reagents: Al, Mg and Ca. They also investigated the influence of milling parameters on the process duration and identified the most optimal parameters for faster kinetics. The planetary mill showed better results than the shaker mill for Mg and Al, while Ca performed well in the SPEX mill.
In depth studies performed by KIT concluded that the process is a mechanically induced self-propagating reaction, which is initiated by mechanical energy, such as the impact energy from the milling balls. Once started, it propagates through the material on its own, similar to a chain reaction. This type of reaction is highly exothermic, releasing a significant amount of heat, which further drives the reaction forward. Small amounts of volatile compounds, such as residual solvents or moisture, were found to have a significant effect on the reaction time. The basis of the volatiles interference is not clear yet, but it seems to be important to remove these compounds before the milling process to ensure a more efficient and faster reaction.
Further experiments are needed to scale up the process and ensure that it can be applied to larger batches.
Vacuum pyrolysis and Supercritical CO2 [scCO2] processing for binders recovery
Binders such as polyvinylidene fluoride [PVDF] and polytetrafluoroethylene [PTFE] for cathodes, and carboxymethyl cellulose [CMC] and styrene-butadiene rubber [SBR] for anodes, are particularly difficult to separate during mechanical processing. This reduces the efficiency of metal recovery and can lead to the formation of environmentally harmful substances, such as per- and polyfluoroalkyl substances [PFAS].
Thermal treatment methods, while effective in removing these organics, generate hazardous emissions like hydrogen fluoride [HF] and phosphoryl fluoride [POF3], necessitating stringent gas treatment measures. Additionally, battery separators, often composed of complex polymers like polyethylene [PE] and polypropylene (PP), pose further challenges due to their low economic value and the presence of residual hazardous materials.
ACC has optimised vacuum pyrolysis at 550°C, which enabled the complete decomposition of PVDF, and thus, the effective removal of binders and separators from LIB waste, while recovering fluorinated compounds from decomposition products. Generated HF was captured using two different methods: through potassium hydroxide [KOH] washing (producing potassium fluoride [KF]) or a calcite filter (producing fluorite).
Supercritical carbon dioxide [scCO₂] process technology has emerged as a promising alternative for LIB recycling, offering properties that are adjustable to organic compounds. However, PVDF dissolution in scCO₂ alone requires extreme conditions, making industrial implementation challenging. Chalmers researchers have explored possible co-solvent scCO₂ systems to enable the recycling of binder directly from mechanically treated BM. They developed a specialised extraction process using scCO₂ with a co-solvent (dimethyl sulfoxide, DMSO) to recover the PVDF binder from industrial battery waste. The process achieved a 55.6 % recovery rate of PVDF under mild conditions.
The scCO₂ technology was also applied to electrolyte and separator recycling, achieving over 90 % separator recovery with high purity.
© visual: ImageFlow via Adobe Stock
There is an undeclared competition for better, more efficient batteries which pushes researchers to continue developing new methods for extracting and synthesising electrodic materials.
Recovery of lithium as battery grade material
Lithium (Li) is a key component in batteries and scientists involved in the RHINOCEROS Project have been exploring ways to extract it from recycled materials from used batteries known as “black mass” (BM). But one of the challenges scientists are facing is the reduction of fluoride content in extracted Li. Researchers at KIT tested their mechanochemical process for extracting Li from various BM samples provided by partners ACC and TES. Their experiments engaging reactive milling coupled with various reactive agents aimed to reduce the fluoride content of the aqueous solution. These tests showed that using magnesium as a reactive agent yielded most promising results for Li extraction.
Progress in Lithium-Manganese battery materials and Advancements in reduced graphene oxide production
Research team at Sapienza Univ. of Rome (UoS) has been making progress in developing lithium-manganese-rich materials for battery applications. These materials were produced using an integrated hydrometallurgical process, which includes the production of reduced graphene oxide and a co-precipitation method that leads to the formation of lithium-manganese-rich cathodes. The resulting cathode materials are currently undergoing extensive physicochemical and electrochemical characterizations.
For the synthesis of reduced graphene oxide, the researchers compared two methodologies and observed differences in productivity. These differences are now being investigated to determine whether they are influenced by the thermal pre-treatment of the graphite or by the role of metals present in different oxidation states. The use of mechano-chemically treated powder has demonstrated remarkable productivity, reaching approximately 80%.
Enhanced solvometallurgical processes
As evoked by its name, solvometallurgy uses solvents to extract metals. Researchers at TEC have been optimising the process, using additives as copper (Cu) or hydrogen peroxide (H2O2) when necessary, achieving a high recovery rate of >95%. However, the process also increased the dissolved Cu content, which required additional steps to reduce it. Researchers are now exploring methods like cementation or electrodeposition to recover and reuse the dissolved Cu. The deep eutectic solvents (DES) were already regenerated and reused, result which could bring a positive impact on the process sustainability assessment.
Direct recovery of battery materials
The Gas-Diffusion Electrocrystallisation (GDEx) technology allows the one-step recovery of metals and synthesis of new materials with high added value. In the framework of the RHINOCEROS project, the research team at VITO has been focusing on optimising the GDEx technology to achieve the selective recovery of nickel (Ni), manganese (Mn), and cobalt (Co), contained in leachates from black mass and achieved 90 % extraction of Ni, Mn and Co. This two-step GDEx process facilitated the removal of all the impurities such as Cu, Fe from the leachate solution. Using the GDEx process, VITO researchers have successfully synthesised Layered Double Hydroxide (LDH) materials and spinel-type nanostructures from the synthetic solutions. The LDH materials were lithiated and LiNi0.8Mn0.1Co0.1O2 (LNMCO811) was synthesised, which could be used as a cathode active material for lithium-ion batteries (LiBs). The results obtained with synthetic solutions portray the potential of the method to obtain relevant active cathode materials out of leachate solutions.
Recovery of materials from low concentration waste streams
Aiming towards a zero-waste strategy for the recovery of metals from battery refining waste waters, LEITAT is working on the development and evaluation of novel polymer inclusion membranes (PIM). PIMs are a type of liquid membrane in which the liquid phase, the extractant, is held within a polymeric network. The interest in these membranes has been growing exponentially over the past few years as an alternative separation technique to conventional solvent extraction.
During the previous six months, the team at LEITAT have been investigating two types of membranes that have shown high selectivity, recovering manganese (Mn) and cobalt (Co) from mixed metal solutions. In their future research, LEITAT will use these membranes in combination to ensure increased selectivity of targeted metals.
Optimising lithium carbonate recovery
Lithium carbonate(Li₂CO₃) is another critical material for batteries, and researchers at TEC and LEITAT are working to optimise its recovery from various solutions. This involves fine-tuning the conditions for Li₂CO₃ precipitation, including the influence of pH and the presence of other cations. Tests are currently conducted with both synthetic solutions and real leachates to ensure the effectiveness of the process. Additionally, efforts are underway to automate the recovery process, which includes assembling elements for pH monitoring and CO2 bubbling systems.
Bringing innovations to market
To bring these innovations to market, researchers are preparing to scale up their processes. This involves a selective process based on data collection, life cycle assessment (LCA) and life cycle cost (LCC) analysis, to ensure the best technological routes are chosen to be further upscaled and meet the production demands.
Author: KIT
During the third semester, researchers from KIT further studied and improved the conditions for the mechanochemical transformation of black mass (BM) into metallic black mass (MBM). Since BM supplied by ACC is already in a reduced state, they focused on reducing BM supplied by TES. This BM consists mostly of NMC (lithium nickel manganese cobalt oxides) cathode material and graphite, which was found to slow down the reaction kinetics. The reduction of the cathode active material by the metallic reducing agent result in the formation of the transition metals along with lithium oxide (Li2O) and the oxide of the respective reducing agent, which can be monitored by X-ray diffraction.
In contrast to the previous two semesters, researchers switched from shaker mills to planetary mills, which enable control of the rotation speed and larger volumes that can be processed. Various parameters such as ball-to-sample ratio (BSR), ball size, total load and rotation speed were investigated to optimise for a short reaction time.
Main take-aways
In general, the higher the BSR, the more mechanical energy can be transferred per gram of powder which results in a more intense milling and a faster reaction; however, this limits the throughput. Larger balls, on the one hand, lead to higher kinetic energies. On the other hand, fewer balls are used to keep the BSR constant resulting in a lower collision frequency. The maximum rotation speed is lower to prevent damage to the grinding media.
With Calcium as the reducing agent, no reaction was achieved at all. An unfavorable combination of ductility and size of the calcium pieces seems to resist further size reduction, which is required for the reaction.
Aluminium has the advantage of being used as a current collector and is already present in the black mass. However, during the reaction, LiAlO2 is formed, which is limiting the subsequent Li extraction efficiency in WP5. This problem can be avoided when magnesium is used as the reducing agent, which proved to be more reactive than aluminium but doesn’t form other lithium compounds than Li2O.
Compared to the shaker mill, a higher reaction rate was observed in the planetary mill. Researcher from KIT achieved a complete conversion of the lithium transition metal oxide in the planetary mill within 3 h using Mg as the reducing agent. In a larger version of the mill, the required milling time increases to 8 hours. Here, further investigations are planned for the next months.
Read previous article on the pre-treatment operations: Pre-treatment operations: Reactive milling for the production of metallic black mass
© Photo: Adobe
The modified Black Mass (BM) obtained in Task 4.4 – Reactive milling for the production of metallic black mass (MBM), as well as the BM supplied by partner ACCUREC (ACC) were leached under different conditions to extract lithium (Li) salt.
The BM provided by ACC consists of graphite, as well as transition metals such as nickel (Ni), manganese (Mn), cobalt (Co), along with their respective oxides and impurities – copper (Cu), iron (Fe), and few fluorinated compounds. Li is present in the form of lithium carbonate [Li2CO3] and lithium aluminium oxide [LiAlO2 ]. However, breaking off LiAlO2 to a soluble Li-salt and removing fluoride contamination is a challenging process. To overcome this, the BM was heated with calcium hydroxide in deionised water for one hour and then filtered.
Illustrated in the next figure, LiAlO2 decomposes during heating and transforms into insoluble calcium aluminium hydroxide. Dissolved fluoride anions are caught by Ca2+ ions and precipitate as calcium fluoride. The soluble part only contains Li- and Ca-salts. The latter can be transformed into calcium carbonate and removed with a second filtration step.
X-ray diffraction patterns of leaching and purification process. a) black mass supplied by ACC and treated with calcium hydroxide. b) aluminium system of modified black mass
The second BM from partner TES, obtained after ball milling, consists of graphite, the metallic composite, lithium oxide and the oxidized reducing agent, along with some impurities like Fe and fluorinated compounds.
Lithium extraction after ball milling
In the aluminium system, the extraction of lithium salt poses certain challenges. Due to the tendency of residual aluminium powder to ignite when in contact with air, this requires cautious handling. During milling lithium aluminium oxide is formed, and in the basic solution, a significant amount of aluminium hydroxide dissolves and reacts with lithium hydroxide and CO2, resulting in the formation of a poorly soluble compound called LACHH. To address this problem, the suspension is heated to 90°C to reinforce the reaction between aluminium hydroxide and lithium hydroxide. The LACHH formed can be decomposed at 350°C into insoluble aluminium oxide and soluble lithium carbonate, which can be separated through filtration.
Lithium extraction in the calcium system, on the other hand, is a relatively straightforward process. The milled powder shows low reactivity towards both oxygen and water. Scientists successfully extracted 98% of lithium from the black mass; however, calcium hydroxide was also dissolved in the process. Subsequent purification steps led to the isolation of lithium carbonate with a purity of 93% in a yield of 75%. The main impurities are shown in the figure displayed below.
In the upcoming months, researchers will work to improve the leaching conditions towards a lower reagent and water consumption, while obtaining a higher purity of resulting lithium salt and lower lithium loses during purification steps.

Impurity cations in isolated lithium carbonate from the calcium system of modified black mass
Focusing on mechanochemical (MC) processing, the chemical transformation of the black masses (BMs) supplied by partners ACCUREC and TES, is planned to be carried out within Work package 4.
Before MC processing, the black masses were analysed using a combination of different analytical techniques. Both quantitative and qualitative analysis were undertaken to determine the Lithium and transition metals yield of the developing recycling process.
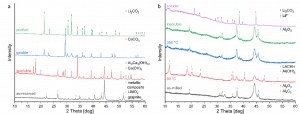
Using different reducing agents such as Al, Ca, and their mixtures, researchers carried out preliminary investigations of the MC processing of BMs. Within this task, different aspects, such as the role of the ball milling conditions, the ball milling time, presence of other nonreactive components, and nature of the reducing material were investigated. The analysis led to the conclusion that the kinetics of the MC-induced reduction reaction is sensitive to multiple processing parameters, as shown in the featured image:

XRD patterns of the as-received BMs and products of their reduction after MC processing with Al and Ca as reducing agents: left-TES material; right-ACC material
The upcoming research will focus on improving the reduction reaction kinetics and eliminating the possible safety hazards of fine powder materials. Once finalised, this work will determine the optimal ball milling conditions to be scaled up.
Recovery of Lithium as battery grade materials
The process described in Task 4.4 leads to the chemical transformation of the black masses (BMs) into ferromagnetic Co-, Ni- and Mn-containing products, which will be separated from other by-products. Lithium will be extracted from the rest of the solid products in the subsequent aqueous leaching process to be further transformed into battery-grade lithium carbonate (Li2CO3) salt.
Within the M1-M6 period, aqueous leaching of the ball-milled samples using Al and Ca as reducing agents (RA) was carried out. At a preliminary stage of investigations, researchers noticed the resulted Li2CO3 materials presented small amount of impurities.
To increase the yield of lithium recycling, in the upcoming period, the research work will target the improvement of the leaching conditions and the purification process.

X-ray diffraction patterns of the as-milled BM obtained from TES, products of their reduction reactions after MC processing with Al (left) and Ca (right) as RA, and obtained Li salts after aqueous leaching
