News
Materials extraction and direct routes for the synthesis of electrode materials
17/04/2024
Author: KIT
Following previous work performed in work package 5, researchers from KIT further investigated the lithium (Li) extraction from black mass (BM) supplied by partners ACCUREC (ACC) and TES.
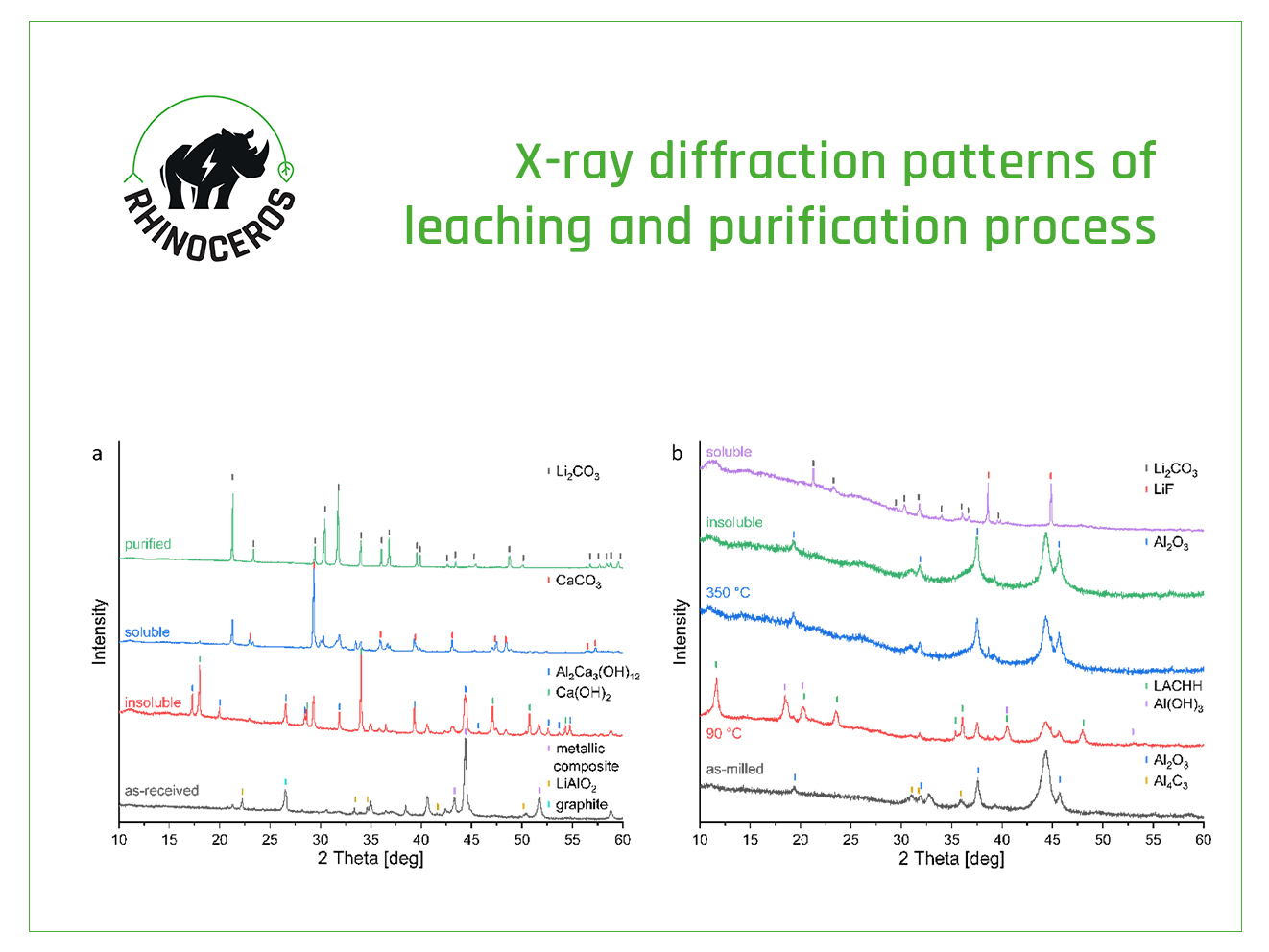
The BM provided by ACC consists of graphite, as well as transition metals such as nickel (Ni), manganese (Mn), and cobalt (Co), along with their respective oxides and impurities like copper (Cu), iron (Fe) and few fluorinated compounds. Li is present in the form of lithium carbonate (Li2CO3), lithium fluoride (LiF) and lithium aluminium oxide (LiAlO2). However, breaking off lithium aluminium oxide to a soluble Li-salt and removing fluoride contamination proved to be challenging.
Previous work reported the simultaneous incorporation of fluoride ions and decomposition of LiAlO2 using an excess of calcium hydroxide (Ca(OH)2) at elevated temperatures. More recent developments of the processes show improvement, especially decreasing considerably the amount of of Ca(OH)2 , while achieving a Li extraction of 87 %. Instead of heating the suspension, it is possible to initiate the reaction by liquid-assisted grinding in a planetary mill. The results show LiAlO2 is decomposed. However, the fluoride content is not efficiently removed.
In the BM supplied by the partner TES, most Li is present as lithium transition metal oxides (LiTMO) and a small amount of LiF. To enable Li extraction, this BM was reduced by reactive milling in Task 4.4 – Reactive milling for the production of metallic black mass (MBM). The obtained MBM consists of graphite, the metallic composite, lithium oxide and the oxidised reducing agent, along with some impurities like Fe and fluorinated compounds.
After investigating aluminium (Al) and calcium (Ca) in the previous steps, researchers at KIT performed tests to assess Li extraction using a magnesium (Mg) system, which presents various advantages, namely: avoiding, on one hand, the formation of Li salts with bad solubility, and relying on the other hand on insolubility of Mg in the high pH aqueous solution.
After aqueous extraction, researchers obtained a mixture of Li2CO3 and LiF. In the upcoming months, this mixture will be purified to a battery-grade material.
News