News
Materials extraction and direct routes for the synthesis of electrode materials: Recovery of Lithium as battery grade materials
29/09/2023
The modified Black Mass (BM) obtained in Task 4.4 – Reactive milling for the production of metallic black mass (MBM), as well as the BM supplied by partner ACCUREC (ACC) were leached under different conditions to extract lithium (Li) salt.
The BM provided by ACC consists of graphite, as well as transition metals such as nickel (Ni), manganese (Mn), cobalt (Co), along with their respective oxides and impurities – copper (Cu), iron (Fe), and few fluorinated compounds. Li is present in the form of lithium carbonate [Li2CO3] and lithium aluminium oxide [LiAlO2 ]. However, breaking off LiAlO2 to a soluble Li-salt and removing fluoride contamination is a challenging process. To overcome this, the BM was heated with calcium hydroxide in deionised water for one hour and then filtered.
Illustrated in the next figure, LiAlO2 decomposes during heating and transforms into insoluble calcium aluminium hydroxide. Dissolved fluoride anions are caught by Ca2+ ions and precipitate as calcium fluoride. The soluble part only contains Li- and Ca-salts. The latter can be transformed into calcium carbonate and removed with a second filtration step.
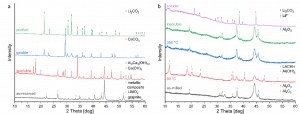
X-ray diffraction patterns of leaching and purification process. a) black mass supplied by ACC and treated with calcium hydroxide. b) aluminium system of modified black mass
The second BM from partner TES, obtained after ball milling, consists of graphite, the metallic composite, lithium oxide and the oxidized reducing agent, along with some impurities like Fe and fluorinated compounds.
Lithium extraction after ball milling
In the aluminium system, the extraction of lithium salt poses certain challenges. Due to the tendency of residual aluminium powder to ignite when in contact with air, this requires cautious handling. During milling lithium aluminium oxide is formed, and in the basic solution, a significant amount of aluminium hydroxide dissolves and reacts with lithium hydroxide and CO2, resulting in the formation of a poorly soluble compound called LACHH. To address this problem, the suspension is heated to 90°C to reinforce the reaction between aluminium hydroxide and lithium hydroxide. The LACHH formed can be decomposed at 350°C into insoluble aluminium oxide and soluble lithium carbonate, which can be separated through filtration.
Lithium extraction in the calcium system, on the other hand, is a relatively straightforward process. The milled powder shows low reactivity towards both oxygen and water. Scientists successfully extracted 98% of lithium from the black mass; however, calcium hydroxide was also dissolved in the process. Subsequent purification steps led to the isolation of lithium carbonate with a purity of 93% in a yield of 75%. The main impurities are shown in the figure displayed below.
In the upcoming months, researchers will work to improve the leaching conditions towards a lower reagent and water consumption, while obtaining a higher purity of resulting lithium salt and lower lithium loses during purification steps.

Impurity cations in isolated lithium carbonate from the calcium system of modified black mass
News




