News
Selective Electrolyte Recovery from spent Li-Ion Batteries using Sub – and Supercritical Carbon Dioxide Technology
25/03/2024
Authors: CHA and PNO
An important component of a Li-ion battery (LiB), the electrolyte has a crucial role in the cell performance. The nonaqueous electrolyte is a multicomponent system consisting of a conductive salt, mainly LiPF6 (Lithium hexafluorophosphate – inorganic component), organic carbonate solvents and additives.
Numerous research initiatives are dedicated to the design of the electrolyte composition, aiming to optimized performance, increased safety, lifetime and streamlined costs. However, once a LiB reaches its end-of-life (EoL) stages, the electrolyte receives less attention in the favour of the valuable metals that can be recycled from the cathode: Li, Co, Mn, Ni, Al, Cu. Moreover, due to its volatility, toxicity and high flammability, the electrolyte recycling is less studied. When ignored, the spent electrolyte reacts with water to form fluoride, leading to its uncontrolled decomposition and/or evaporation, and thus generating an imminent environmental risk. Due to its composition that includes organic solvents, the presence of residual electrolyte in the black mass is considered hazardous, and it is often a reason hampering the recycling process.
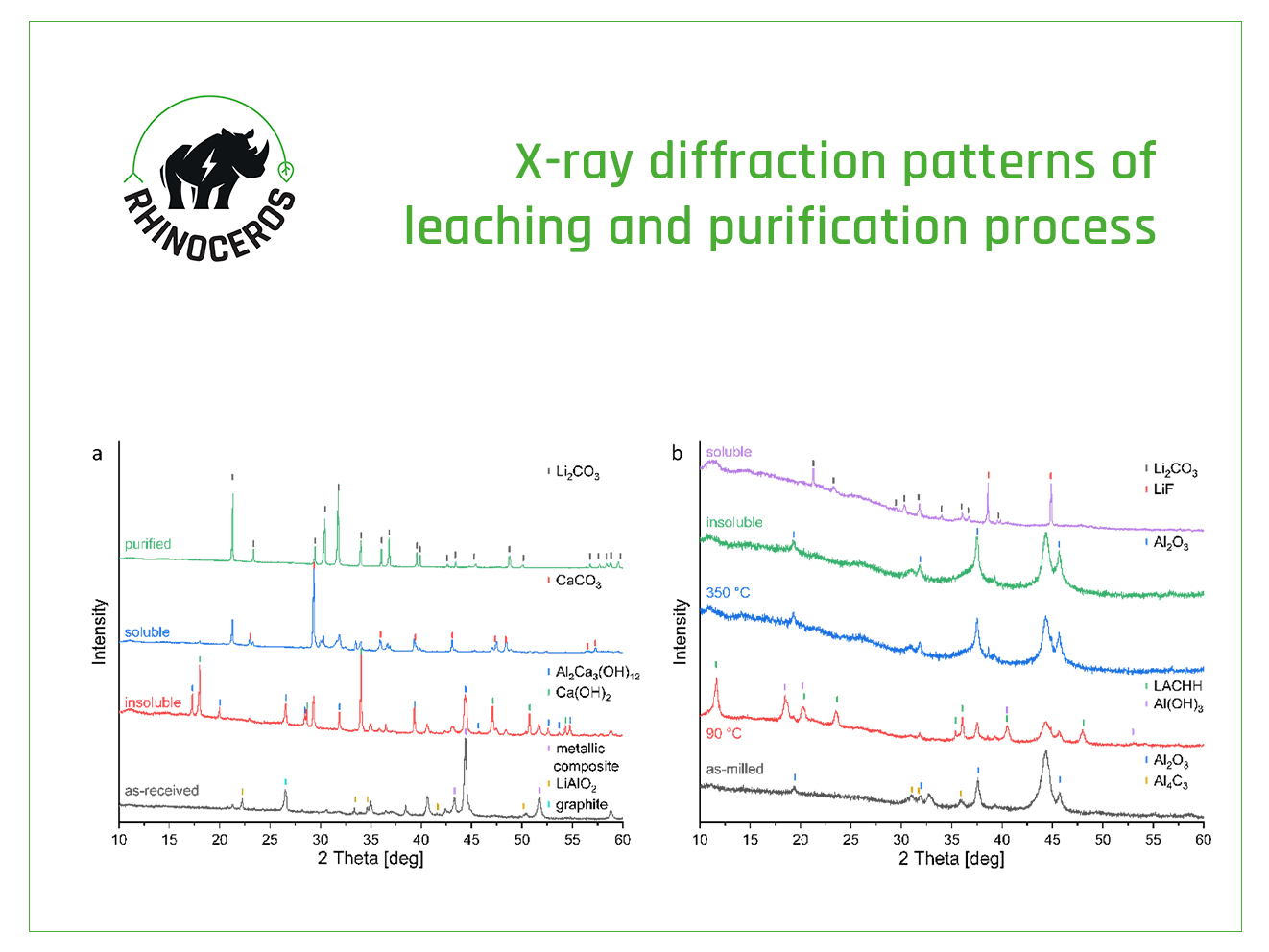
Researchers at Chalmers University (CHA) ) developed a sub- and supercritical carbon dioxide (sc-CO2) extraction process to selectively recover the electrolyte from spent LiBs. Sc-CO2 extraction technologies are already widely employed in the food, beverage, pharmaceutical, and cosmetic industry. In a nutshell, sc-CO2 forms when CO2 surpasses its critical point at 31°C and 73.8 bar pressure. In this so-called supercritical state, CO2 demonstrates optimal mass-transfer characteristics and can be fine-tuned to alter its physicochemical characteristics by adjusting pressure and/or temperature. However, CO2 proves ineffective as a solvent for high molecular weight polymers and highly polar ionic compounds. However, the addition of a co-solvent or a modifier can significantly improve the solubility properties of sc-CO2, making this alternative a suitable process to selectively extract LiPF6 after solvent removal.
Researchers at CHA investigated different process parameters (pressure, temperature, extraction times) to understand their impact on the extraction behavior of different electrolyte solvents such as ethylene carbonate. Their analysis included both qualitative and quantitative results indicating the composition and the purity. Simultaneously, the research also monitored the process exhaust stream for potential formation of LiPF6 decomposition products. The findings showed that that the non-polar electrolyte solvents like dimethyl carbonate, diethyl carbonate, and ethyl methyl carbonate were successfully extracted using low-density CO2. The more polar electrolyte components such as ethylene carbonate and propylene carbonate were stepwise extracted by gradually increasing the system’s pressure. The developed technology is a game changer not only for electrolyte recycling but also for increased workplace and transportation safety due to removal of the flammable and hazardous substances from battery black mass. The simplicity of the processing design is an additional advantage of the technology.
© Photo credit: Adobe
News