News
Electrochemical extraction of Lithium from end-of-life Li-ion batteries as a possible strategy for LiOH recovery
22/03/2024
During the past years, the increase in the use of lithium-ion batteries (LIBs) has become more prominent. An unsurprising trend, whatsoever, due to the widespread and rapid adoption of clean mobility applications, electronic devices, and energy storage systems. Despite their undeniable environmental and social benefits, several challenges lie ahead. In 2022, global lithium demand exceeded the supply despite an 180% increase, IEA reports. Recent communications already forecast the demand of lithium (Li) is expected to soar over the next decade, with mobility accounting for the main consuming market.
The current supply of primary resources is deemed insufficient for the growing demand. Approximately 60% of today’s lithium is mined for battery-related applications, a figure that could reach 95 percent by 2030, McKinsey reports estimate. But this rapid increase in the use of LIBs in EVs will introduce a large quantity of spent batteries in the near future. The alternatives to manage spent batteries include remanufacturing, repurposing and recycling, with the latter one playing a significant role from both ecologic and economic points of view.
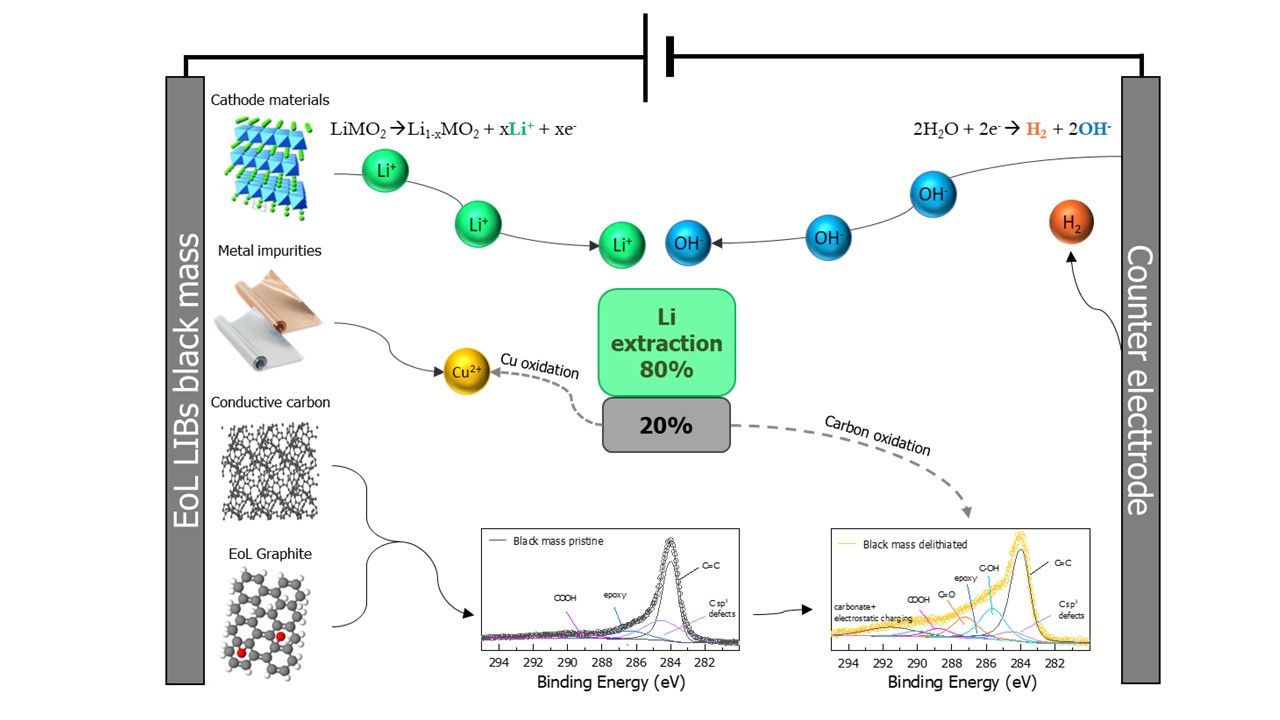
Current recycling processes lack selectivity in recovery control and require significant consumption of reagents and energy. The research conducted by the RHINOCEROS partners at Sapienza University of Rome (UoS), Department of Chemistry, aims to develop an electrochemical process for selective extraction of Li from electrodic powder of end-of-life (EoL) LIBs. This concept simulates the charging process of a LIB with an aqueous electrolyte and a cathode material (counter electrode) that facilitates water reduction. The hydroxyls freed by water reduction and the Li + cations deintercalated by the anode will form a LiOH solution.
Using two samples – a commercial powder, respectively one coming from EoL LIBs, and testing the delithiation process using various parametres, UoS researchers obtained:
- Li extraction of 99% from commercial powder
- Li extraction of 82% from waste powder
The lower Li extraction on EoL electrode powders compared to commercial ones is due to the presence of SuperP [a high purity and structured carbon black powder with a moderate surface area for the lithium-ion industry], which oxidises under delithiation conditions.
Discover the scientific publication
News