News
Author: KIT
During the third semester, researchers from KIT further studied and improved the conditions for the mechanochemical transformation of black mass (BM) into metallic black mass (MBM). Since BM supplied by ACC is already in a reduced state, they focused on reducing BM supplied by TES. This BM consists mostly of NMC (lithium nickel manganese cobalt oxides) cathode material and graphite, which was found to slow down the reaction kinetics. The reduction of the cathode active material by the metallic reducing agent result in the formation of the transition metals along with lithium oxide (Li2O) and the oxide of the respective reducing agent, which can be monitored by X-ray diffraction.
In contrast to the previous two semesters, researchers switched from shaker mills to planetary mills, which enable control of the rotation speed and larger volumes that can be processed. Various parameters such as ball-to-sample ratio (BSR), ball size, total load and rotation speed were investigated to optimise for a short reaction time.
Main take-aways
In general, the higher the BSR, the more mechanical energy can be transferred per gram of powder which results in a more intense milling and a faster reaction; however, this limits the throughput. Larger balls, on the one hand, lead to higher kinetic energies. On the other hand, fewer balls are used to keep the BSR constant resulting in a lower collision frequency. The maximum rotation speed is lower to prevent damage to the grinding media.
With Calcium as the reducing agent, no reaction was achieved at all. An unfavorable combination of ductility and size of the calcium pieces seems to resist further size reduction, which is required for the reaction.
Aluminium has the advantage of being used as a current collector and is already present in the black mass. However, during the reaction, LiAlO2 is formed, which is limiting the subsequent Li extraction efficiency in WP5. This problem can be avoided when magnesium is used as the reducing agent, which proved to be more reactive than aluminium but doesn’t form other lithium compounds than Li2O.
Compared to the shaker mill, a higher reaction rate was observed in the planetary mill. Researcher from KIT achieved a complete conversion of the lithium transition metal oxide in the planetary mill within 3 h using Mg as the reducing agent. In a larger version of the mill, the required milling time increases to 8 hours. Here, further investigations are planned for the next months.
Read previous article on the pre-treatment operations: Pre-treatment operations: Reactive milling for the production of metallic black mass
© Photo: Adobe
The modified Black Mass (BM) obtained in Task 4.4 – Reactive milling for the production of metallic black mass (MBM), as well as the BM supplied by partner ACCUREC (ACC) were leached under different conditions to extract lithium (Li) salt.
The BM provided by ACC consists of graphite, as well as transition metals such as nickel (Ni), manganese (Mn), cobalt (Co), along with their respective oxides and impurities – copper (Cu), iron (Fe), and few fluorinated compounds. Li is present in the form of lithium carbonate [Li2CO3] and lithium aluminium oxide [LiAlO2 ]. However, breaking off LiAlO2 to a soluble Li-salt and removing fluoride contamination is a challenging process. To overcome this, the BM was heated with calcium hydroxide in deionised water for one hour and then filtered.
Illustrated in the next figure, LiAlO2 decomposes during heating and transforms into insoluble calcium aluminium hydroxide. Dissolved fluoride anions are caught by Ca2+ ions and precipitate as calcium fluoride. The soluble part only contains Li- and Ca-salts. The latter can be transformed into calcium carbonate and removed with a second filtration step.
X-ray diffraction patterns of leaching and purification process. a) black mass supplied by ACC and treated with calcium hydroxide. b) aluminium system of modified black mass
The second BM from partner TES, obtained after ball milling, consists of graphite, the metallic composite, lithium oxide and the oxidized reducing agent, along with some impurities like Fe and fluorinated compounds.
Lithium extraction after ball milling
In the aluminium system, the extraction of lithium salt poses certain challenges. Due to the tendency of residual aluminium powder to ignite when in contact with air, this requires cautious handling. During milling lithium aluminium oxide is formed, and in the basic solution, a significant amount of aluminium hydroxide dissolves and reacts with lithium hydroxide and CO2, resulting in the formation of a poorly soluble compound called LACHH. To address this problem, the suspension is heated to 90°C to reinforce the reaction between aluminium hydroxide and lithium hydroxide. The LACHH formed can be decomposed at 350°C into insoluble aluminium oxide and soluble lithium carbonate, which can be separated through filtration.
Lithium extraction in the calcium system, on the other hand, is a relatively straightforward process. The milled powder shows low reactivity towards both oxygen and water. Scientists successfully extracted 98% of lithium from the black mass; however, calcium hydroxide was also dissolved in the process. Subsequent purification steps led to the isolation of lithium carbonate with a purity of 93% in a yield of 75%. The main impurities are shown in the figure displayed below.
In the upcoming months, researchers will work to improve the leaching conditions towards a lower reagent and water consumption, while obtaining a higher purity of resulting lithium salt and lower lithium loses during purification steps.

Impurity cations in isolated lithium carbonate from the calcium system of modified black mass
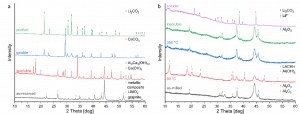
Focusing on mechanochemical (MC) processing, the chemical transformation of the black masses (BMs) supplied by partners ACCUREC and TES, is planned to be carried out within Work package 4.
Before MC processing, the black masses were analysed using a combination of different analytical techniques. Both quantitative and qualitative analysis were undertaken to determine the Lithium and transition metals yield of the developing recycling process.
Using different reducing agents such as Al, Ca, and their mixtures, researchers carried out preliminary investigations of the MC processing of BMs. Within this task, different aspects, such as the role of the ball milling conditions, the ball milling time, presence of other nonreactive components, and nature of the reducing material were investigated. The analysis led to the conclusion that the kinetics of the MC-induced reduction reaction is sensitive to multiple processing parameters, as shown in the featured image:

XRD patterns of the as-received BMs and products of their reduction after MC processing with Al and Ca as reducing agents: left-TES material; right-ACC material
The upcoming research will focus on improving the reduction reaction kinetics and eliminating the possible safety hazards of fine powder materials. Once finalised, this work will determine the optimal ball milling conditions to be scaled up.
Recovery of Lithium as battery grade materials
The process described in Task 4.4 leads to the chemical transformation of the black masses (BMs) into ferromagnetic Co-, Ni- and Mn-containing products, which will be separated from other by-products. Lithium will be extracted from the rest of the solid products in the subsequent aqueous leaching process to be further transformed into battery-grade lithium carbonate (Li2CO3) salt.
Within the M1-M6 period, aqueous leaching of the ball-milled samples using Al and Ca as reducing agents (RA) was carried out. At a preliminary stage of investigations, researchers noticed the resulted Li2CO3 materials presented small amount of impurities.
To increase the yield of lithium recycling, in the upcoming period, the research work will target the improvement of the leaching conditions and the purification process.

X-ray diffraction patterns of the as-milled BM obtained from TES, products of their reduction reactions after MC processing with Al (left) and Ca (right) as RA, and obtained Li salts after aqueous leaching
