News
Supercritical CO2 technology for recycling spent LIBs
16/03/2023
The current industrial pre-treatment and downstream processes (e.g., pyrolysis, calcination, etc.) are still inefficient and have significant limitations. Plastics and electrolytes sacrificed in the initial stages of the recycling process are overlooked when it comes to their recovery. Within the RHINOCEROS project, Chalmers University [CHA] is working on recycling of ignored content of the LIBs waste, electrolyte and polymeric materials by developing an innovative process based on Supercritical Carbon Dioxide (sc-CO2) technology.
Due to its environmental friendliness, non-toxic, low cost and straightforward processing features, the sc-CO2 technology has been attracting both scientific and industrial interest. With consistent leverage over other processes, sc-CO2 technology is already widely used in various industries including food, cosmetic and pharmaceutical industries [i.e., to decaffeinate coffee or tea, extract vegetable oils etc.] Although it has many applications, its use in battery recycling was recently discovered and the Chalmers research group is one of the pioneers in this field.
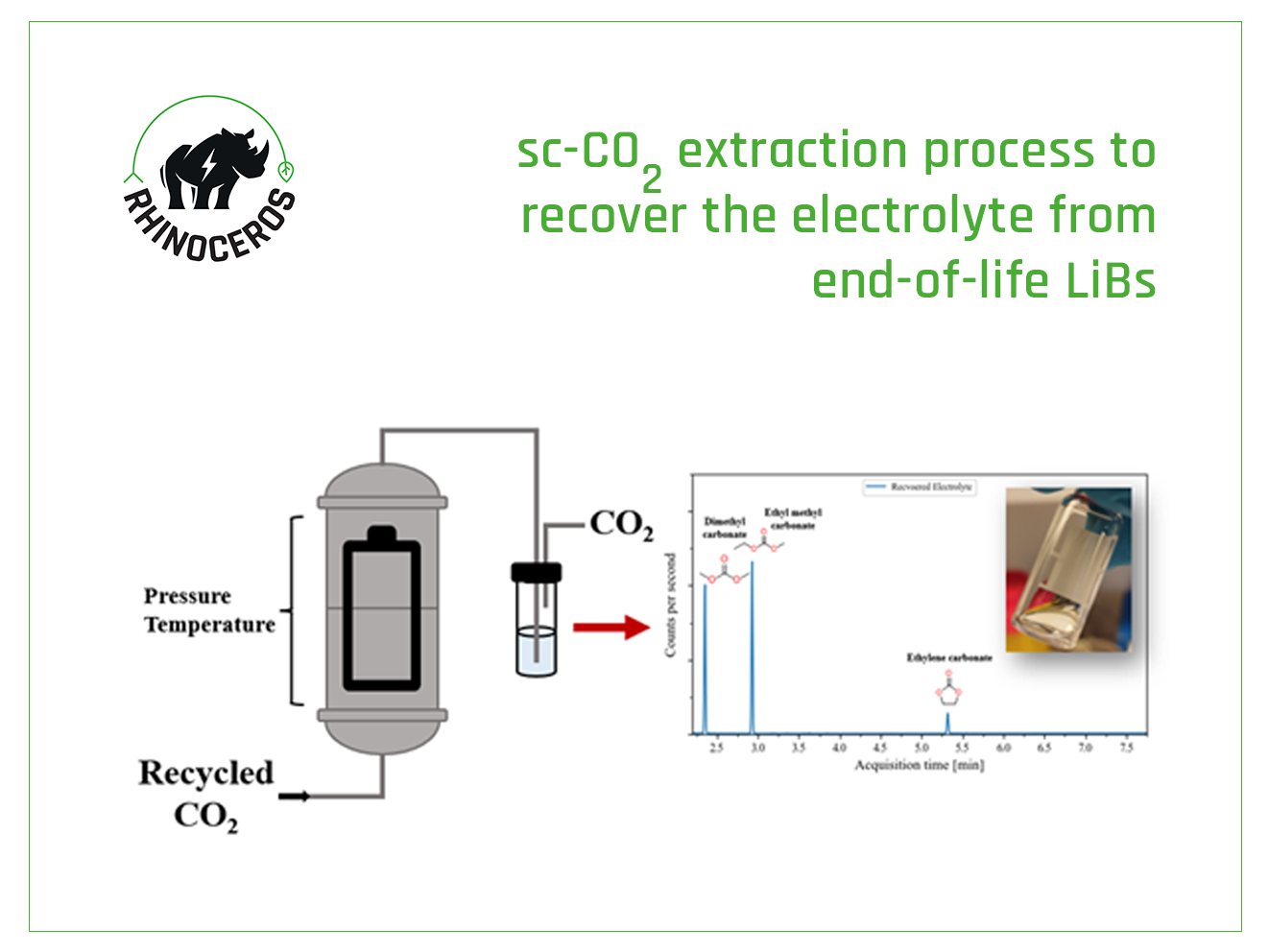
Within the project’s framework, CHA researchers are targeting the development of the sc-CO2 extraction process which will selectively recycle the electrolyte and the polymeric material from the LiB waste. The electrolyte, binder, and separator will be recycled in subsequent steps and purified to reuse in the battery industry. For this purpose, several critical process parameters such as pressure and temperature are investigated to achieve high recycling efficiencies under feasible conditions.
Electrolyte recovery
The electrolyte in the LiB is a complex system usually composed of a conductive salt dissolved in a matrix of various solvents and additives. The most recent results reported by CHA on sub- and sc-CO2 research show that at low pressure and temperature conditions, the non-polar electrolyte components (almost 66% of the electrolyte) were selectively recovered without the generation of toxic gas emissions, which are typically generated by thermal recovery processes originated by the decomposition of the thermally unstable conductive salt.
In this recycling step, the polar electrolyte components are left in the sample as residues and a subsequent recycling step using suitable cosolvent is required for their selective extraction. During the upcoming phases, researchers will aim to recover the electrolyte completely, including conductive salt and solvents.
In this recycling step, the polar electrolyte components are left in the sample as residues and a subsequent recycling step using suitable cosolvent is required for their selective extraction. During the upcoming phases, researchers will aim to recover the electrolyte completely, including conductive salt and solvents.
The selection of the co-solvent is critically important not only for effective recycling but also for the sustainability and the feasibility of the developed processes. To assess the suitability of the sc-CO2 process, its environmental impact and economic competitiveness, CHA researchers explored also other solvents, which allowed them to select the most promising candidates for future research.
In the upcoming months, CHA will study the effects of co-solvent modified sc-CO2 system parameters on the recycling efficiency of PVDF and the structural properties of the recycled material. Researchers will carry out intensive characterisation studies to clarify the changes in the material structure, to determine the quality, and to optimise the process to reach the reusability goal.
News




